David Atwood
University of Alabama, B.S. Chemistry (1987)
University of Texas at Austin, Ph.D. Chemistry (1992)
Overview
We conduct research that unites fundamental studies with real-world applications in the general areas of environmental chemistry, metal chelate compounds, and solid-state materials. The research combines aspects of inorganic, organic, analytical, biological, and physical chemistry and consequently utilizes a broad range of analytical techniques. Examples of these techniques are listed in the sidebar, Laboratory Techniques. Much of the research is collaborative and conducted with students and faculty at other institutions and with scientist in industry and national laboratories.
For a more detailed research description please see the sidebar: Research Description.
Inorganic, Organometallic, and Solid-State Materials Synthesis and Characterization Laboratory Techniques (see sidebar)
Main Group Chemistry, Founder (1996) and Editor-in-Chief. This quarterly, peer-reviewed journal publishes articles which focus on the main group (s and p blocks) and lanthanide elements. The articles span a wide range of fundamental and applied chemistry subjects including biology, catalysis, environmental, inorganic, materials, medicine, organometallics, pharmaceuticals, and toxicology. http://www.iospress.nl/journal/main-group-chemistry
Inorganic Chemistry: A Wiley Textbook Series, Editorial Board. This is a series of textbooks designed for instructional purposes. 18 textbooks, designed for instructional use, have been published in this series. Some recent titles include: Mass Spectrometry of Inorganic and Organometallic Compounds: Tools-Techniques-Tips (2005) William Henderson and J. Scott McIndoe, Lanthanide and Actinide Chemistry (2006) Simon Cottton, Inorganic Structural Chemistry, 2nd Edition (2006) Ulrich Muller, Bioinorganic Vanadium Chemistry (2008) Dieter Rehder, Chirality in Transition Metal Chemistry: Molecules, Supramolecular Assemblies and Materials (2009) Hani Amouri and Michel Gruselle, Introduction to Coordination Chemistry (2010) Geoffrey A. Lawrance. http://www.wiley.com/WileyCDA/Section/id-302900.html
Encyclopedia of Inorganic and Bioinorganic Chemistry (EIBC), Editorial Board. EIBC was formed by combining the two major reference works, The Encyclopedia of Inorganic Chemistry and the Handbook of Metalloproteins. EIBC now has over 10,000 pages of reference information in articles that are updated regularly. The resource is available in print and online. http://onlinelibrary.wiley.com/book/10.1002/9781119951438
Edited Reference Works:
1. Radionuclides in the Environment (2010) 522 pages. http://www.wiley.com/WileyCDA/WileyTitle/productCd-0470714344.html
2. The Rare Earths: Fundamentals and Applications (2012) 696 pages. http://www.wiley-vch.de/publish/en/books/forthcomingTitles/CH00/1-119-9…

Other Volumes in this series: Applications of Physical Methods to Inorganic and Bioinorganic Chemistry (2007) Editors: Robert A. Scott and Charles M. Lukehart, Nanomaterials: Inorganic and Bioinorganic Perspectives (2008) Editors: Charles M. Lukehart and Robert A. Scott, Computational Inorganic and Bioinorganic Chemistry (2009) Editors: Edward I. Solomon, Robert A. Scott, and R. Bruce King, Energy Production and Storage: Inorganic Chemical Strategies for a Warming World (2010) Robert H. Crabtree
Edited Books
1. Group 13 Chemistry, From Fundamentals to Applications, P. J. Shapiro and D. A. Atwood, Eds., The American Chemical Society, Washington, DC, 2002.
2. Group 13 Chemistry I: Fundamental New Developments, Editors: H. W. Roesky and D. A. Atwood, Structure & Bonding, Vol. 103, Springer-Verlag, Berlin, 2002.
3. Group 13 Chemistry II: Biological Aspects of Aluminum, Editors: H. W. Roesky and D. A. Atwood, Structure & Bonding, Vol. 104, Springer-Verlag, Berlin, 2002.
4. Group 13 Chemistry III: Industrial Applications, Editors: H. W. Roesky and D. A. Atwood, Structure & Bonding, Vol. 105, Springer-Verlag, Berlin, 2002.
5. Recent Developments in Mercury Science, Editor: D. A. Atwood, Structure and Bonding, Vol. 120, Springer-Verlag, Berlin, 2006.
Interests: Inorganic and Materials Chemistry, Organic and Organometallic Chemistry, Biological and Pharmaceutical Chemistry
Techniques: Vacuum Line and Glovebox handling of air-sensitive compounds, Chromatography (TLC, GC, HPLC, LCMS, IC), Circular Dichroism (CD), Cyclic Voltammetry (CV), Differential Scanning Calorimetry (DSC), Direct Mercury Analysis (DMA), Fluorescence Spectroscopy, Graphite Furnace Atomic Absorption Spectroscopy (GFAA), Inductively-Coupled Plasma Optical Emission Spectroscopy (ICP-OES), Infrared Spectroscopy (IR), Magnetic Susceptibility, Microscopy (SEM, TEM), Nuclear Magnetic Resonance (NMR), Raman Spectroscopy, Thermogravimetric Analysis (TGA), Karl Fischer Water Determination, Single-Crystal X-ray Diffractometry (SCXRD), UV-Vis Spectroscopy, X-ray Absorption Fine Structure (XAFS), X-ray Absorption Near-Edge Spectroscopy (XANES), X-Ray Fluorescence (XRF), X-ray Powder Diffration (XRPD).
1. D. A. Atwood, “Reactions at a Group IA Metal Center," Inorganic Reactions and Methods, Vol. 14, Section 10.2.2, Wiley-VCH, Inc., New York, 1998; 115-131.
2. D. A. Atwood, "Oxidative Addition and Reductive Elimination Reactions at a Group IVB Metal Center," Inorganic Reactions and Methods, Vol. 14, Section 10.2.5, Wiley-VCH, Inc., New York, 1998; 153-178.
3. D. A. Atwood, "Preparation of the Nitrides of Al, Ga and In," Inorganic Reactions and Methods, Vol. 18, Sections 17.3.8.2 - 17.3.8.4, Wiley-VCH, Inc., New York, 1998; 222-229.
4. M. J. Harvey and D. A. Atwood, "Chelated Aluminum Anions," in Group 13 Chemistry, From Fundamentals to Applications, P. J. Shapiro and D. A. Atwood, Eds., ACS Symposium Series, Vol. 822, American Chemical Society, Washington, D.C., 2002, pp. 131-141.
5. B. D. Conley, U. Dutta, C. Fridh, A. L. Gilliam, B. C. Yearwood, J. P. Selegue and D. A. Atwood, "Chemistry of the Tetrafluoroaluminate Anion" in Group 13 Chemistry I, From Fundamentals to Applications, P. J. Shapiro and D. A. Atwood, Eds., ACS Symposium Series, Vol. 822, American Chemical Society, Washington, D.C., 2002, pp. 259-270.
6. B. Conley and D. A. Atwood, "Fluoroaluminate Chemistry" in Group 13 Chemistry II: Biological Aspects of Aluminum, H. Roesky and D. A. Atwood, Eds., Structure and Bonding, Vol. 104, Springer-Verlag, Berlin, 2002, pp. 181-194.
7. D. A. Atwood, A. H. Hutchison, Y. Wang, "Compounds Containing Five-Coordinate Group 13 Elements," Group 13 Chemistry III: Industrial Applications, H. Roesky and D. A. Atwood, Eds., Structure and Bonding, Vol. 105, Springer-Verlag, Berlin, 2002, pp. 167-201.
8. D. A. Atwood, M. K. Zaman, “Mercury Removal From Water”, Recent Developments in Mercury Science, D. A. Atwood, Ed., Structure and Bonding, Vol. 120, Springer-Verlag, Berlin, pp. 163-182
9. A. Mitra and D. A. Atwood, "Phosphate Ester Cleavage with Binuclear Boron Chelates," in Modern Aspects of Main Group Chemistry, M. Lattman and R. A. Kemp, Eds., ACS Symposium Series, Vol. 917, American Chemical Society, Washington, DC, 2006, pp. 390-409.
Research Description
The research we conduct unites fundamental studies with real-world applications in the general areas of environmental chemistry, metal and metalloid chelate compounds, and solid-state materials. The research combines aspects of inorganic, organic, analytical, biological, and physical chemistry and consequently utilizes a broad range of analytical techniques. Examples of these techniques are listed in the sidebar, Research Techniques. Much of the research is collaborative and conducted with students and faculty at other institutions and with scientist in industry and national laboratories. (Some representative references are provided; the numbers (#) refer to the publication list on this website under Academics.)
I. Environmental Chemistry
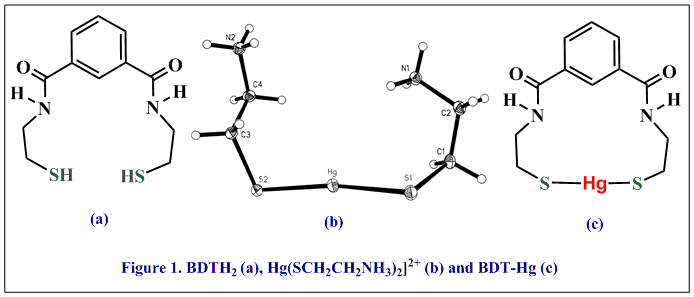
The synthetic dithiol compound, BDTH2*, has emerged as an exceptional reagent for removing mercury and other soft divalent metals from water through the formation of covalent metal-sulfur bonds (Figure below). BDTH2 has good thermal stability, solubility in a wide range of organic solvents, does not easily form disulfide bonds, and has no odor. BDTH2 has sufficient solubility in water (400 – 2000 parts-per-million, ppm) to address most aqueous metal-contamination problems. BDTH2 has the remarkable ability, when used in a 10% excess, to precipitate Cd, Hg, and Pb from water to below detectable limits (low parts-per-billion, ppb). Furthermore, the BDT-metal precipitates are impervious to leaching except under extremely acidic or basic conditions. The covalent sulfur-metal bonds in the BDT-metal compounds are exceedingly stable and release metal only under extremely acidic and basic conditions (#161, Fuel 89 (2009) 1326). *The abbreviation “BDTH2” is derived from the common name “BenzeneDiamidoEthaneThiol” (IUPAC nomenclature, N,N’-Bis(2-mercaptoethyl)isophthalamide).
BDTH2 irreversibly precipitates mercury under a wide range of laboratory conditions, from groundwater on-site at former chlor-alkali facilities, contaminated soils outside a natural gas pressure-monitoring station (#129), and from gold mining effluent at pH 12 (#114, Env. Sci. Technol. 36 (2002) 1636 and #124, Ind. Eng. Chem. Res. 42 (2002) 5278. BDTH2 precipitates a variety of divalent metals from acid mine drainage (#120) and lead from lead-battery recycling effluent (#113, Ind. Eng. Chem. Res. 41 (2002) 1579). Arsenic has been of particular interest over the past few years. We have conducted field studies of agricultural lands where arsenic-containing poultry litter has been applied and we’ve prepared of a filtration unit capable of removing arsenite from tap water.
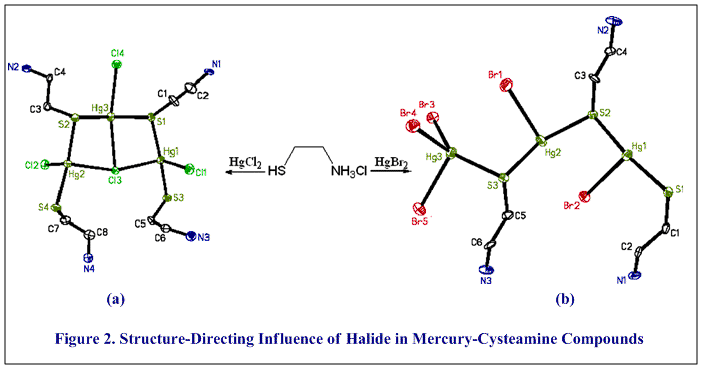
Continuing research is elucidating the fundamental properties of BDTH2 and developing and understanding of the structure, bonding, and reactivity of metals and metalloids with BDTH2. Derivatives of BDTH2, and new compounds distinct from BDTH2, are being designed with properties targeted for specific industrial applications. Cysteamine, a component of the BDTH2 molecule, is a biological molecule and related to the amino acid, cysteine. Mercury forms linear compounds with cysteamine and cysteine (Figure 1(b); #109, Polyhedron 40 (2002) 225), but compounds with cysteamine and heavy metal halides have very diverse, multinuclear structures (Cd: #134; Pb: #146; Hg: #141, #149, Inorg. Chem. 45 (2006) 2112 and #150, Inorg. Chem. 45 (2006) 7261). The type of halide has a structure-directing influence in the mercury compounds (Figure 2; #140, Inorg. Chem. 44 (2005) 5753, #141, #149,Inorg. Chem. 45 (2006) 2112, #150, Inorg. Chem. 45 (2006) 7261). We use structures like these to better understand the toxicity, mobility, and speciation of metals and metalloids in biological systems.
Water purification technologies that we or others develop need to have the capability of detecting and quantifying the contaminants being removed. For example, a household water filtration system that removes arsenic or heavy metals should be able to “turn-off” when the filter has reached capacity or when the filter has been compromised or does not perform properly. To address this need we have partnered with Quansor, Inc. to develop new sensors for the real time detection of inorganic, biological, and organic contaminants in water. The proprietary Quansor Monitor system has the capability of determining the levels of specific contaminants as the water is passing through the system, in “real-time”. The Quansor Monitor is designed so that household or industrial water-quality information can be transmitted wirelessly to a computer or cell phone. We are specifically responsible for the sensor chemistry which we can immediately test and demonstrate on the Quansor Monitor installed in our laboratory.
II. Metal and Metalloid Chelate Compounds
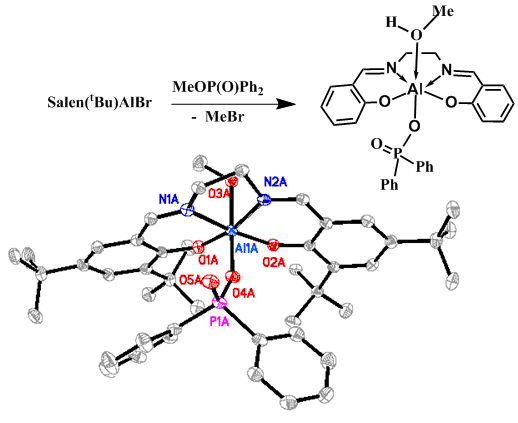
Over the past two decades we have developed an extensive series of group 13 chelate compounds (Chem. Rev. 101 (2001) 37), many of which are cationic, that are catalysts for oxirane and lactide oligomerization and polymerization (#110, J. Chem. Soc. Dalton Trans. (2002) 410). The two most common structures for the compounds are binuclear (salhen(tBu)[BBr2]2) and mononuclear (salen(tBu)AlBr) (Figure 3) where salen is a tetradentate chelate). We have made a wide variety of metal and metalloid salen compounds in different stoichiometries and with different structures. Indeed, there really are no limitations to what a student could work on in this area by varying the type of ligand, the ligand substituents, metal or metalloid, reaction stoichiometry, and conditions. For example, we’ve made chelate compounds with the Group 2 elements in liquid ammonia and zinc compounds with the salen ligand derivatized to be water-soluble as potential CO2 capture agents (an extension of our much earlier work with zinc chelates; Inorg. Chim. Acta 277 (1998) 157).
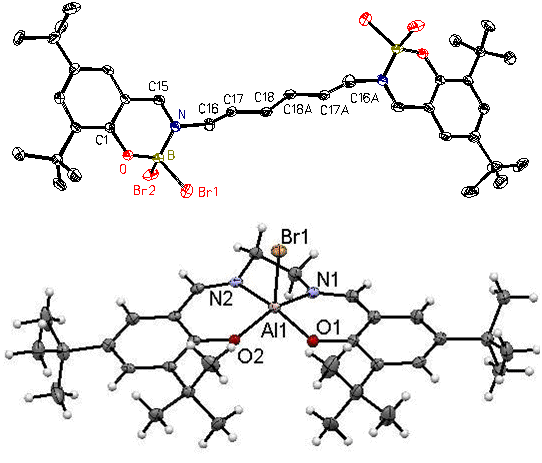 |
| Figure 3. The Crystal Structures of Salhen(tBu)(BBr2)2 (above) and Salen(tBu)AlBr (below) |
Two specific projects will serve as examples of the research we are now conducting in this area. The first is the characterization of the monomeric, oligomeric and polymeric aluminum chelate-containing compounds that result from phosphate ester dealkylation (Section A). In the second project we use aluminum chelates to deactivate nerve agents and pesticides through dealkylation (Section B and example in Figure 4). Studies on actual nerve agents and pesticides are conducted by collaborators at the Edgewood Chemical and Biological Center. We use non-toxic, much less harmful nerve agent and pesticide simulants and model compounds at UK. The ultimate goal of this project is to create water-soluble compounds that can decontaminate objects, such as vehicles or aircraft and their interior areas, by using the compound like soap and water (Figure 5).
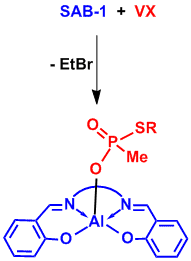 |
 |
|
Figure 4. Deactivation of Nerve Agent VX through Dealkylation and Covalent Bonding. (SAB = Salen Aluminum Bromide) |
Figure 5. Water-Soluble Derivative of Aluminum Chelate (SAB-1) for the Decontamination of Objects Exposed to Organophosphate Chemical Weapons.
|
A. Aluminum Chelate Compounds through Dealkylation
In the course of exploring the chemistry of chelated boron cations we discovered a unique reaction where salenAlX compounds (X = Cl and Br) induced cleavage of all three alkyl groups in trialkyl phosphates, (RO)3P=O (#112, J. Am. Chem. Soc. 124 (2002) 1864). Subsequently, we determined that the mono-metallic aluminum chelates, and in particular, the salenAlBr compounds had the most utility in dealkylating a broad range of phosphates in short periods of time (#142, J. Am. Chem. Soc. 128 (2006) 1147). We were quickly able to confirm the identity of the first two compounds, (a) and (b), in the proposed reaction sequence (Figure 6).
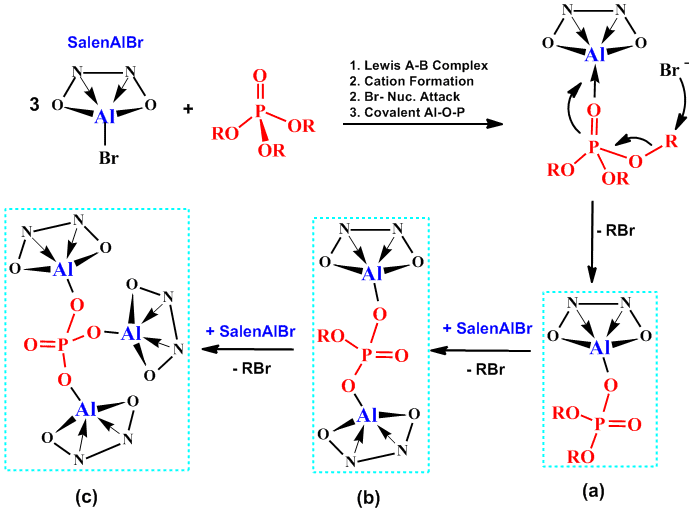 |
| Figure 6. Complete Dealkylation of Trialkyl Phosphate to Produce Three New Inorganic Aluminum Phosphates (a), (b), and (c). |
One example of compound (a) is the first structurally-characterized terminal aluminum phosphinate (Figure 7; #147, Inorg. Chem. 45 (2006) 3970). We had previously established that aluminum-phosphinates form dimeric and polymeric compounds (# 111, Inorg. Chem. 41 (2002) 558) but in this example the bulky salen(tBu) ligand and a moderately sterically encumbered phoshphinate led to the isolation of the unique monomer. Our current goals are to isolate further examples of the twice-dealkylated products (b), and possibly of greater interest, the fully dealkylated product (c). These compounds should be soluble in organic solvents suitable for characterization by X-ray crystallography and would be a new class of inorganic aluminum phosphate. However, it is unlikely that the structures of the compounds will be as simple as represented in Figure 6(c) with three very bulky salen(tBu)Al units surrounding a central phosphate. Rather, the resulting compounds could have extended structures and will probably be ionic. Whatever new structures we discover will certainly posses unique bonding and reactivity.
|
|
| Figure 7. Single Dealkylation of Phosphinate to Produce Terminal Aluminum Phosphate and the Crystal Structure of the Compound. |
B. Nerve Agent Deactivation
We have demonstrated that the dealkylation reaction can be employed to dealkylate problematic nerve agents and environmental contaminants such as pesticides, both which possess phosphate ester (P-O-C) bonds (#142, J. Am. Chem. Soc. 128 (2006) 1147 and #156, New J. Chem. 32 (2008) 783). For nerve agents such as Sarin a key feature of the reaction is that the phosphate ester is cleaved to leave a covalent B-O-P or Al-O-P linkage in the final product (Figures 4 and 5 above). Thus, the breakage of a single bond allows for the deactivation of the entire chemical weapon agent, obviating the need for further treatment of the by-products. This makes the technology imminently suitable for use in gas masks where the compounds can be introduced adsorbed onto activated carbon, for example. For bulk chemical weapons destruction the dealkylation reaction can be made catalytic by the addition of BBr3, to regenerate the B-Br or Al-Br bonds and release the decomposed nerve agent as a covalent boron or aluminum phosphate (#112, J. Am. Chem. Soc. 124 (2002) 1864). For a review of chemical weapon agent destruction and detection see #165, Chem. Rev. 111 (2011) 5345.
IV. Solid-State Materials
Molecular precursors containing oxophilic metals can be converted to nanoparticulate metal oxides through ambient hydrolyses. This takes place due to the pre-existing metal-oxygen bonds that preordain the conversion of the molecule into the metal-oxide material (Section A). This “soft” materials synthesis technique is being explored for the preparation of nanomaterials with molecular functionality on the surface, and to create a wide variety of “mixed-metal” oxide materials with transition metals (Section B.) and lanthanides (Section C). Additionally, understanding metal-oxygen bonding provided a new means for reducing the oxidation of molten aluminum during secondary aluminum production (Section D).
A. Molecular Precursors to Nanoparticulate Alumina
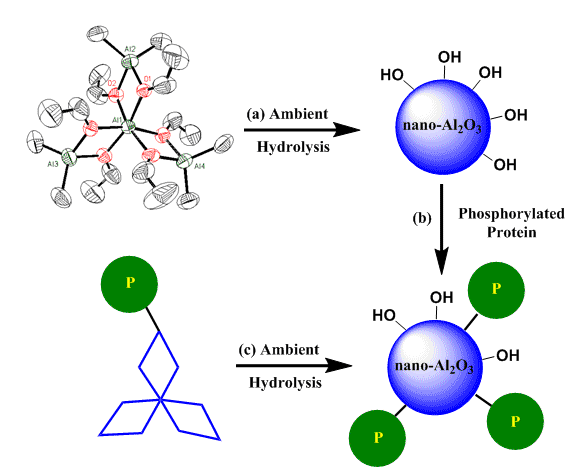
We have created a new class of tetradentate compounds having the appropriate Al:O stoichiometry for the formation of Al2O3 (Organomet. 18 (1999) 976). The molecules have a tri-diamond shape that looks like the emblem of the Mitsubishi™ Company and so we refer to them as “Mitsubishi™ Molecules”(Coord. Chem. Rev. 210 (2000) 1). The compounds hydrolyze in organic solvents open to air to produce nanoparticulate alumina (#135, Z. Anorg. Allg. Chem. 631 (2005) 2937). In this reaction the Mitsubishi™ molecule acts as a template for the formation of alumina (Figure 8(a)), rather than the hydrated forms of alumina that results when organoaluminum compounds are hydrolyzed. In collaboration with an industrial sponsor we demonstrated that the compounds deposit a corrosion resistant covering layer of alumina on heated metal substrates under anaerobic conditions.
We have used the nanoparticulate alumina to create an alumina-Pepsin composite material (Figure 7(b)) that is heterogeneous but maintains the activity found in the native enzyme (#126, Nano Lett. 3 (2003) 55). However, the alumina-Pepsin composite is easier to handle and isolate and is more thermally stable than the native enzyme. Alumina nanoparticles with attached biological components could be used in clinical diagnostics, nanosensors, localized delivery of biopharmaceuticals, and biological recognition systems. Furthermore, the “soft” ambient hydrolysis reaction may allow the introduction of the molecular functionality into the precursor molecule before the formation of the nanomaterial (Figure 7(c)). The resulting particle sizes could be controlled through changes in solvent, concentration, and temperature. We are exploring the potential of this methodology for the preparation of metal oxide-biological composite materials with transition metals and lanthanide elements. The materials would combine the biological function with the metal-based magnetic, luminescent, and other unique properties.
|
|
| Figure 8. Ambient Hydrolysis of a "Mitsubishi™ Molecule" to Nanoparticulate Alumina (Al2O3) (a), Derivatization of the Nanoparticles with Phosphorylated Pepsi (b), and Derivatization of the Mitsubishi™ Molecule with Subsequent Hydrolysis and Formation of Nanoparticles with Surface Functionality (c). |
B. Mixed-Metal Compounds
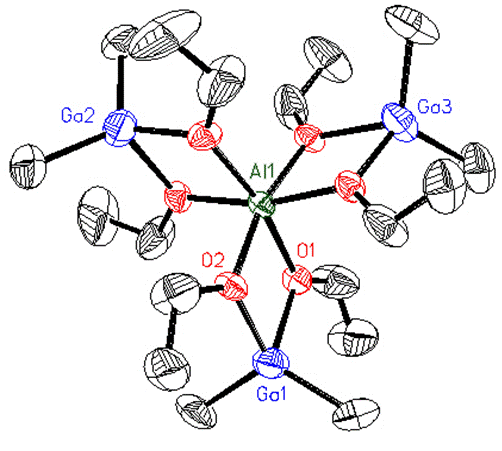
Gallium can be introduced into the structure of a Mitsubishi™ molecule (Figure 9(a)). Gallium atoms are slightly smaller than aluminum, due to the d-orbital contraction, and occupy the peripheral four-coordinate sites in the structure with the larger aluminum atom in the central six-coordinate site. This compound could be used as a single-sources precursor to nanoparticulate AlxGayOz materials if it undergoes hydrolytic decomposition like the all-aluminum compounds (Organomet. 18 (1999) 976).
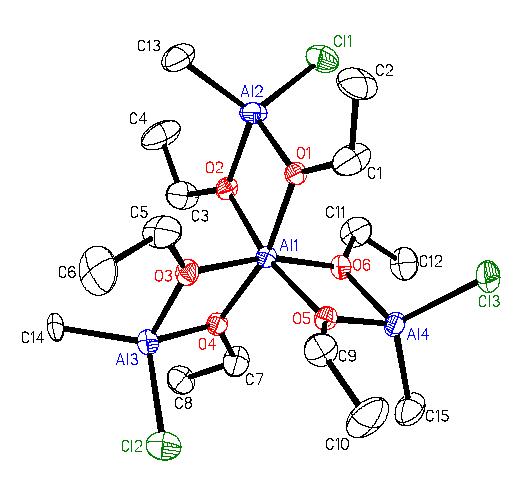
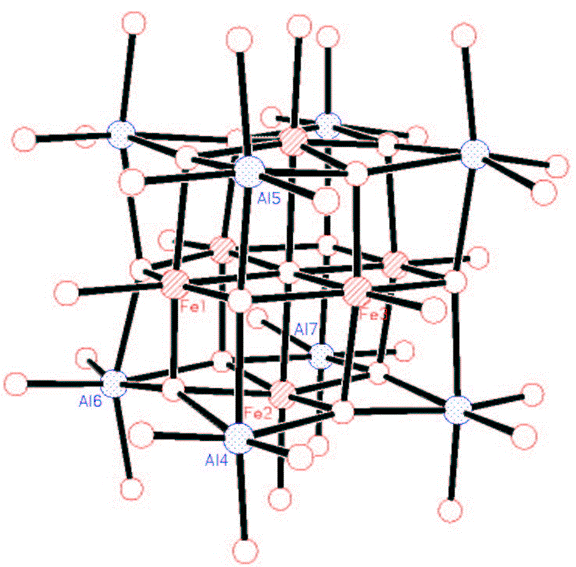
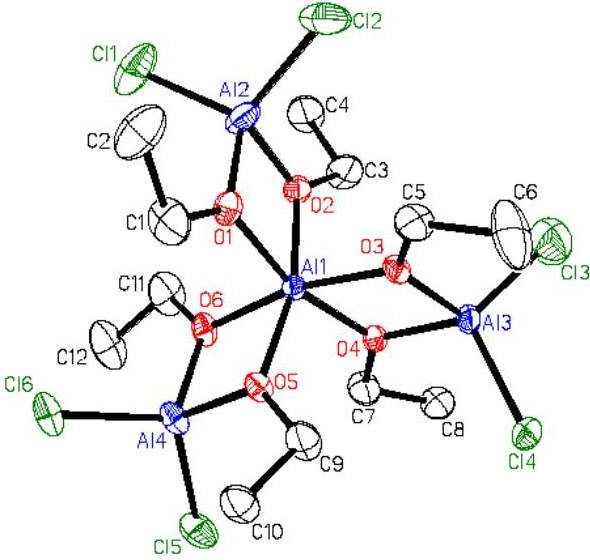
We have also made Mitsubishi™ molecules that have one or two surface chloride groups (Figures 9(b) and 9(c))#155, J. Chem. Soc. Dalton Trans. (2008) 1037). We have combined the halide-containing compounds with transition metal anions with the expectation that a salt elimination reaction will take place to form compound where the transition metal is coordinated by the oxygens of the Mitsubishi™ molecule. In the partial crystal structure (terminal groups are disordered Cl and Me) of the resulting compound the aluminum and iron atoms are bridged by oxygen atoms in a lattice that looks like it could be easily converted into the AlxFeyOz solid state material (Figure 9(c)).
|
|
|
|
|
 |
C. Molecular Precursors to Lanthanide-Aluminum Oxides
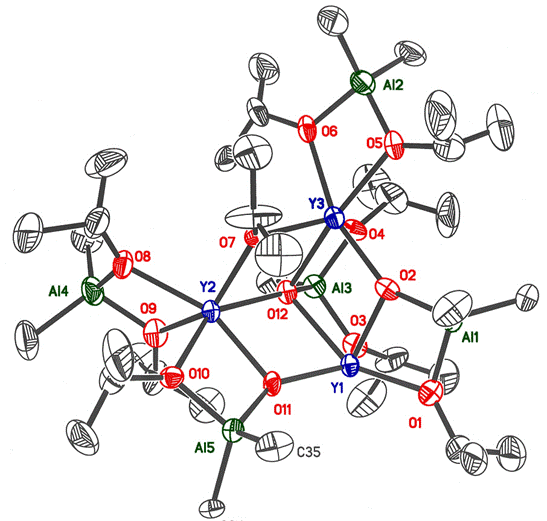
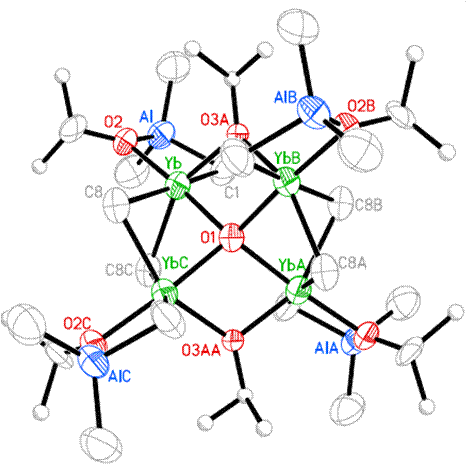
Combination of AlMe3 with M(OiPr)3 forms the structurally characterized compounds shown below (with M = Y (left) and Yb (right); (#136, Main Group Chemistry 4 (2005) 3). When the compounds are hydrolyzed under ambient conditions an amorphous solid state material forms. The materials are likely to be nanoparticulate but further characterization has not been attempted. Heating of the solids causes the formation of crystalline Yb3Al5O12 and Y3Al5O12 (YAG). These reactions indicate the possibility that almost any molecular precursor containing oxophilic metals can be hydrolyzed to new or metastable phases of aluminum-metal-oxide materials. The low, possibly ambient, temperatures needed to cause hydrolysis would be conducive to the formation of nanoparticulate materials and metastable material phases.
 |
 |
D. Prevention of Dross Formation in Molten Aluminum
In secondary aluminum refining, such as the recycling of aluminum cans (made from Al-Mg alloys), molten aluminum (mp 667°C) is oxidized at a relatively slow rate until a point is reached where accelerated “break-away” oxidation occurs resulting in loss of up to ~50% of the aluminum metal as dross (aluminum oxides). The break-away oxidation occurs when there is sufficient MgAl2O4 (spinel) present to act as a conduit for oxygen into the subsurface of the molten aluminum. Spinel forms through cation replacement in the solid MgO (mp 2852°C) present in the melt. Literature from several decades ago revealed that adding small amounts of boron (and beryllium) reagents to molten aluminum dramatically inhibited the formation of aluminum oxide (dross). The boron also reduced the amount of spinel that formed on the surface of the molten aluminum. In our own experiments ppm levels of boric acid were sufficient to dramatically reduce the oxidation of aluminum. We hypothesized that the added boron acts to prevent the transformation of MgO to MgAl2O4 by forming one or more covalent B-O-Mg bonds to the corners of the MgO crystallites. This would account for the ability of such low amounts of boron to prevent “break-away” aluminum oxidation. It would take only eight boron atoms connected to the corners of an MgO cube by B-O-Mg covalent bonds to prevent the entire cube of MgO from converting into spinel (#163, Main Group Chem. 9 (2010) 193 and Book Chapter 11). This covalent surface coating effect” would also explain how our synthetic dithiol compounds prevented metal leaching from sulfide minerals (# 154, Main Group Chem. 6 (2007) 169).
Please see: https://ens.as.uky.edu/
Description of Contents
I. "Rare Earths: Resource Sustainability". Abstract of an article to be published in The Rare Earth Elements: Fundmentals and Applications (October 2012). The article describes the issues related to the unsustainable consumption of the Rare Earths (the lanthanide elements). This is not just a problem of resource depletion. The unique properties of the Rare Earth elements are the basis of a multitude of routine, daily-use products, and are irreplacable components in existing and emerging Green Energy technologies.
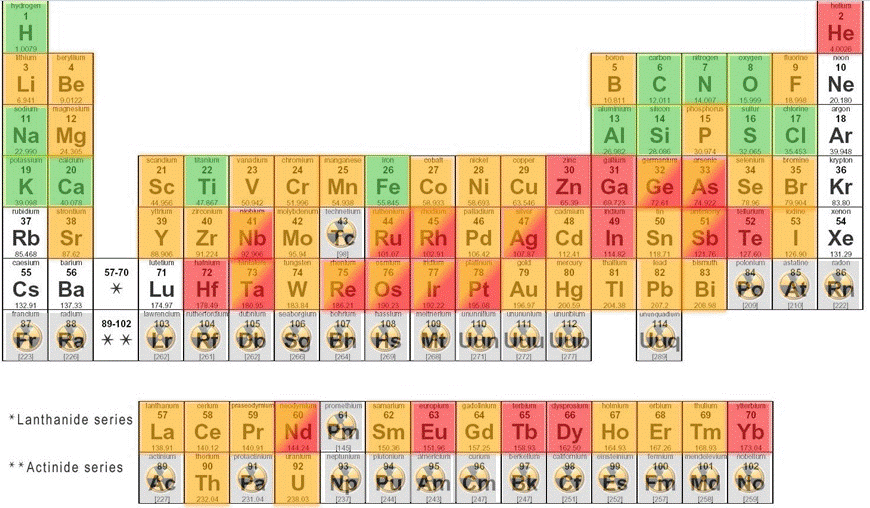
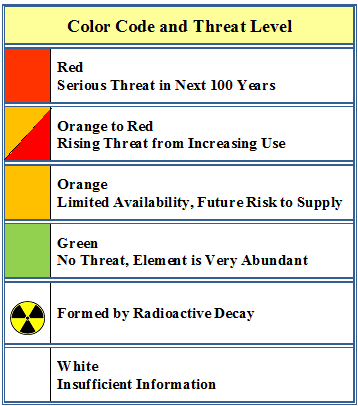
II. Endangered Elements Periodic Table. This version of the Periodic Table provides an assessment of the of the elements that will be in short supply, "endangered", over the next 100 years. The Periodic Table was produced by Mike Pitts, Director of Sustainability at Chemistry Innovation (https://connect.innovateuk.org/web/mike-pitts/summary). Chemistry Innovation is a knowledge-sharing network provided by Open Innovation _connect. The _connect website provides access to a wide range of networks related to science, technology, and industry (https://connect.innovateuk.org/web/guest/home).
To obtain a copy of the Endangered Elements Periodic Table (shown below) and to get more information on this subject see: http://connect.innovateuk.uat.technophobia.com:8081/web/sustainability-…
III. Publications about Endangered Elements
A. "Let's take better care of our rare earth elements", Mike Pitts, New Scientist, Issue 2799, February 2011. (The Article is attached below). Also available at: http://www.newscientist.com/article/mg20927995.700-lets-take-better-car…
B. "Endangered Elements" Chemistry World, Critical Thinking Section, Emma Davies, Subheading: "As our supply of some essential elements dries up, it's time to start urban mining"}, January 2001. (The Article is attached below). Also available at: https://docs.google.com/viewer?a=v&q=cache:u1paElaMeT0J:www.rsc.org/ima…
C. "Endangered Elements", The Chemical Engineer, Mike Pitts, October 2011 (This is a great article on the subject but it does't appear to be accessible throuhg UK).
IV. Environmental Sustainability Knowledge Transfer Network (Sustainability and Chemistry). This is a network in the _connect organization. It provides information and guidance to chemistry-using industries (not just the "chemical industry") in four key priority areas: 1) Sustainable Water Management, 2) Sustainable Energy, 3) Resource Efficiency, and 4) Sustainable Land Management and Food Production. https://connect.innovateuk.org/web/sustainabilityktn/overview
____________________________________________________________________________________
I. "Rare Earths: Resource Sustainability"
Chapter in The Rare Earth Elements: Fundamentals and Applications, October 2012
David A. Atwood, Department of Chemistry, University of Kentucky, Lexington KY, 40506-0055; datwood@uky.edu
Abstract
Rare earth elements have unique electronic, optical, luminescent, and magnetic properties that make them critically important in a broad range of products and applications. For example, rare earth elements are used as catalysts, in manufacturing, medicine, ceramics, and glasses. They are fundamentally important in the generation of clean energy and carbon-free means of transportation. Projected growth rates of rare earth element demands will, for some elements, exceed production capabilities within several decades. Moreover, rare earth elements are currently mined, concentrated, and used in an unsustainable manner that will make these critically important elements unavailable for future generations. Achieving rare earth sustainability requires a careful economic assessment and a determination of environmental and societal impacts. New chemical and engineering technologies will be required to achieve this goal, beginning with the recycling and reuse of the many products that currently utilize rare earth elements. Ultimately, products and applications should be designed so that rare earth elements can be immediately and economically reused.
Keywords: Sustainability, Strategic Elements, Endangered Elements
Article Contents:
- Overview
- Introduction
- Endangered Elements
- Resources and Consumption
- Markets, Products, Applications
- Reduced Use and Recycling
- Fate and Disposition
- Conclusions
- Future Outlook
- Abbreviations and Acronyms
- Glossary
- References (listed here)
- McDonough, W., and Braungart, M. “Cradle to Cradle: Remaking the Way We Make Things”, North Point Press, New York, 2002.
- Kingsnorth D. J. “Rare Earths Supply Security: Dream or Possibility” Industrial Minerals Company of Australia (IMCOA) Presentation, April 2012, available online at: https://connect.innovateuk.org/c/document_library/get_file?p_l_id=34914… (Accessed May 5, 2012).
- The role of REE in clean energy technology was the subject of a recent U.S. Senate Hearing: Rare Earths, Hearing before the Subcommittee on Energy of the Committee on Energy and Natural Resources, United States Senate, 111th Congress, 2nd Session, To Examine the Role of Strategic Minerals in Clean Energy Technologies and Other Applications, as well as Legislation to Address the Issue, Including S. 3521, The Rare Earths Supply Technology and Resources Transformation Act of 2010; September 30, 2010 (62-707 PDF).
- D. J. Hanson, “Rare Earths for Security”, Chemical & Engineering News, December 2011; pp 33-34.
- https://connect.innovateuk.org/web/mike-pitts/blogs/-/blogs/update-to-t… (Accessed May 5, 2012).
- E. Davies, Endangered Elements, Chemistry World, 2011, January, 50-54.
- United States Geological Survey, 2011 Mineral Yearbook and Mineral Commodity Summary, http://minerals.usgs.gov/minerals/ (Accessed April 20, 2012).
- A very detailed forecast of REE supply and demand can be found in: E. Alonso, A. M. Sherman, T. J. Wallington, M. P. Everson, F. R. Field, R. Roth and R. E. Kirchain, Evaluating Rare Earth Availability: A Case Study with Revolutionary Demand from Clean Technologies, Environ. Sci. Technol. 2012, 46, 3406-3414.
- T. G. Goonan, Rare Earth Elements-End Use and Recylability, United States Geological Survey Scientific Investigations Report 2011-5094.
- Rare Earth Elements, British Geological Survey, Natural Environment Research Council, November 2011, p. 21.
- J. Kemsley, Metal Recycling Falls Short Chemical & Engineering News, May 30, 2011; p 9.
- T. E. Graedel, J. Atwood, J.-P. Birate, B. K. Reck, S. F. Sibley, G. Sonnemann, M. Buchart, C. Hagelüken, Recycling Rates of Metals: A Status Report, United Nations Environmental Programme (UNEP) 2011, A Report of the Working Group on the Global Metal Flows to the International Resource Panel.
- J.-F. Tremblay, Managing a Dearth of Rare Earths, Chemical & Engineering News, April 2, 2012, pp 14-15.
- M. Pitts, “Let’s Take Better Care of our Rare Earth Elements” New Scientist, February 15, 2011, Issue 2799, pp 26-27.
II. Endangered Elements Periodic Table
Publications from 2002 to Present
108. A. R. Hutchison and D. A. Atwood, "Research with First- and Second-Year Undergraduates: A New Model for Undergraduate Inquiry at Research Universities," J. Chem. Ed., 79, 125-126 (2002).
109. C.-H. Kim, S. Parkin, M. Bharara and D. Atwood, "Linear Coordination of Hg(II) by Cysteamine," Polyhedron, 40, 225-228 (2002).
110. M.-A. Muñoz-Hernández, M. L. McKee, T. S. Keizer, B. C. Yearwood and D. A. Atwood, "Six-Coordinate Aluminum Cations: Characterization, Catalysis, and Theory," J. Chem. Soc., Dalton Trans., 410-414 (2002).
111. Y. Wang, S. Parkin and D. A. Atwood, "Ligand-Tetrahydrofuran Coupling in Chelated Aluminum Phosphinates," Inorg. Chem., 41, 558-565 (2002).
112. T. S. Keizer, L. J. De Pue, S. Parkin and D. A. Atwood, "Catalytic Dealkylation of Phosphates with Binuclear Boron Compounds," J. Am. Chem. Soc., 124, 1864-1865 (2002).
113. M. M. Matlock, B. S. Howerton and D. A. Atwood, "Chemical Precipitation of Lead from Lead Battery Recycling Plant Wastewater," Ind. Eng. Chem. Res., 41, 1579-1582 (2002).
114. M. M. Matlock, B. S. Howerton, M. A. Van Aelstyn, F. L. Nordstrom and D. A. Atwood, "Advanced Mercury Removal from Gold Leachate Solutions Prior to Gold and Silver Extraction: A Field Study from an Active Gold Mine in Peru," Env. Sci. Tech., 36, 1636-1639 (2002).
115. M. M. Matlock, K. R. Henke and D. A. Atwood, "Effectiveness of Commercial Reagents for Heavy Metal Removal from Water with New Insights for Future Chelate Designs," J. Haz. Mat., B92, 129-142 (2002).
116. B. Yearwood, S. Parkin and D. A. Atwood, "Synthesis and Characterization of Organotin Schiff Base Chelates," Inorg. Chim. Acta, 333, 124-131 (2002).
117. M. Sánchez, T. S. Keizer, S. Parkin, H. Höpfl and D. A. Atwood, "Salen-Supported Dinuclear and Trinuclear Boron Compounds," J. Organomet. Chem., 654, 36-43 (2002).
118. P. A. Iyere, W. Y. Boadi, R. S. Brooks, D. Atwood and S. Parkin, "4,4'-Bipyridin-1-ium Bromide Monohydrate," Acta Cryst., E58, 825-827 (2002).
119. B. D. Conley, B. C. Yearwood, S. Parkin and D. A. Atwood, "Ammonium Hexafluorosilicate Salts," J. Fluor. Chem., 115, 155-160 (2002).
120. M. M. Matlock, B. S. Howerton and D. A. Atwood, "Chemical Precipitation of Heavy Metals from Acid Mine Drainage,"Wat. Res., 36, 4757-4764 (2002).
121. T. S. Keizer, L. J. De Pue, S. Parkin and D. A. Atwood, "Boron Halide Chelate Compounds and Their Activity towards the Demethylation of Trimethylphosphate," Can. J. Chem., 80, 1463-1468 (2002).
122. T. S. Keizer, L. J. De Pue, S. Parkin and D. A. Atwood, "Salen Supported Molecular Borosilicates," J.Cluster Sci., 13, 609-620 (2002).
123. M. Sánchez, M. J. Harvey, F. Nordstrom, S. Parkin and D. A. Atwood, "Salen-Type Compounds of Calcium and Strontium," Inorg. Chem., 41, 5397-5402 (2002).
124. M. M. Matlock, B. S. Howerton, J. D. Robertson and D. A. Atwood, "Gold Ore Column Studies with a New Mercury Precipitant," Ind. Eng. Chem. Res., 41, 5278-5282 (2002).
125. T. Otieno, A. R. Hutchison, M. K. Krepps and D. A. Atwood, "Synthesis and Spectral and Thermal Properties of Pyrazine-Bridged Coordination Polymers of Copper(II) Nitrate: An Experiment for Advanced Undergraduates," J. Chem. Ed., 79, 1355-1357 (2002).
126. J. Li, J. Wang, V. G. Gavalas, D. A. Atwood and L. G. Bachas, "Alumina-Pepsin Hybird Nanoparticles with Orientation-Specific Enzyme Coupling," Nano Lett., 3, 55-58 (2003).
127. T. S. Keizer, L. J. De Pue, S. Parkin and D. A. Atwood, "Dealkylation with Boron Bromide Chelates," J. Organomet. Chem., 666, 103-109 (2003).
128. M. M. Matlock, B. S. Howerton and D. A. Atwood, "Irreversible Binding of Mercury from Contaminated Soil," Adv. Env. Res., 7, 347-352 (2003).
129. M. M. Matlock, B. S. Howerton and D. A. Atwood, "Covalent Coating of Coal Refuse to Inhibit Leaching," Adv. Env. Res., 7, 495-501 (2003).
130. M. M. Matlock, B. S. Howerton, M. Van Aelstyn, K. R. Henke and D. A. Atwood, "Soft Metal Preferences of 1,3-Benzenediamidoethanethiol," Water Res., 37, 579-584 (2003).
131. A. R. Hutchison and D. A. Atwood, "Mercury Pollution and Remediation: The Chemist's Response to a Global Crisis," J. Chem. Cryst., 33, 631-645 (2003).
132. P. A. Iyere, W. Y. Boadi, R. S. Brooks, D. Atwood and S. Parkin, "Supramolecular Aggregation in 4,4'-Bipyridin-1,1'-ium Dichloride, 4,4'-Bipyridin-1,1'-ium Dinitrate and 4,4'-Bipyridin-1'-ium Bromide," Acta Cryst., B59, 664-669 (2003).
133. Y. Wang, S. Bhandari, S. Parkin and D. A. Atwood, "Five-Coordinate Organoaluminum Acetylides and Crystal Structure of the Hydrosylate, [Salophen(tBu)Al]2O," J. Organomet. Chem., 689, 759-765 (2004).
134. M. S. Bharara, C. H. Kim, S. Parkin and D. A. Atwood, "Synthesis and X-Ray Crystal Structures of Dinuclear Hydrogen-Bonded Cadmium and Lead 2-Aminoethanethiolates," Polyhedron, 24, 865-871 (2005).
135. Y. Wang, S. Bhandari, A. Mitra, S. Parkin, J. Moore and D. A. Atwood, "Ambient-Condition nano-Alumina Formation Through Molecular Control," Z. Anorg. Allg. Chem., 631, 2937-2941 (2005).
136. S. Liu, P. Wei, Y. Wang, E. Santillan-Jimenez, R. C. Bakus II and D. A. Atwood, “Use of a Structurally Characterized Molecular Cluster to Form Yb3Al5O12 Under Ambient Conditions” Main Group Chem., 4, 3 - 10 (2005).
137. A. Mitra and D. A. Atwood, First Example of a Borate-Bridged Dimeric Aluminum Schiff-Base Complex Containing Five-Coordinate Metal Centers, Main Group Chem., 4, 91-96 (2005).
138. A. R. Hutchison, A. Mitra and D. A. Atwood, “The Four-Coordinate Geometric Parameter: A New Quantification of Geometry for Four-Coordinate Aluminum and Gallium” Main Group Chem., 4, 187-200 (2005).
139. M. S. Bharara, S. Parkin and D. A. Atwood, “Solution Behavior of Hg(II)-cystamine by UV-Vis and 199Hg NMR” Main Group Chem., 4, 217-225 (2005)
140. A. Mitra, P. Wei and D. A. Atwood, “Tripodal Saltren(Al(iBu)(Cl))3” Main Group Chem., 4, 309-314 (2005).
141. M. S. Bharara, T. H. Bui, S. Parkin, D. A. Atwood, “Structure-Directing Influence of Halide in Mercury Thiolate Clusters” Inorg. Chem, 44, 5753-5760 (2005).
142. M. S. Bharara, T. H. Bui, S. Parkin, D. A. Atwood, “Mercurophilic Interactions in Polynuclear Hg(II)-2-aminothiolates” J. Chem. Soc. Dalton Trans., 3874-3880 (2005).
143. A. Mitra, L. J. DePue, S. Parkin and D. A. Atwood, "Five-Coordinate Aluminum Bromides: Synthesis, Structure, Cation Formation, and Cleavage of Phosphate Ester Bonds," J. Am. Chem. Soc., 128, 1147-1153 (2006).
144. A. Mitra, M. J. Harvey, M. K. Proffitt, L. J. DePue, S. Parkin and D. A. Atwood, "Binuclear Salan Borate Compounds with Three-Coordinate Boron Atoms," J. Organomet. Chem., 691, 523-528 (2006).
145. A. R. Hutchison and D. A. Atwood, "A Novel Tin (II) Dithioether Complex," J. Organomet. Chem., 691, 1658-1660 (2006).
146. T. A. Shaikh, R. C. Bakus II, S. Parkin and D. A. Atwood, "Structural Characteristics of 2-Halo-1,3,2-Dithiarsenic Compounds and Tris-(pentafluorophenylthio)-arsen," J. Organomet. Chem., 691, 1825-1833 (2006).
147. M. S. Bharara, S. Parkin and D. A. Atwood, "Two-Dimensional Network Acquired by Pb(II) 2-Aminoethanethiolate," Inorg. Chim. Acta, 359, 3375-3378 (2006).
148. A. Mitra, S. Parkin and D. A. Atwood, "Aluminum Phosphinate and Phosphates of Salen Ligands," Inorg. Chem., 45, 3970-3975 (2006).
149. T. A. Shaikh, S. Parkin and D. A. Atwood, "Synthesis and Characterization of a Rare Arsenic Trithiolate with an Organic Disulfide Linkage and 2-Chloro-benzo-1,3,2-dithiastibole," J. Organomet. Chem., 691, 4167-4171 (2006).
150. M. S. Bharara, S. Parkin, D. A. Atwood, “Solution and Solid-State Study of Heteroleptic Hg(II)-Thiolates: Crystal Structures of [Hg4I4(SCH2CH2NH2)4] and [Hg4I8(SCH2CH2NH3)2]n·nH2O,” Inorg. Chem, 45, 2112-2118 (2006).
151. M. S. Bhara, S. Parkin, D. A. Atwood, “Mercury(II) 2-Aminoethanethiolate Clusters: Intramolecular Transformations and Mechanisms,” Inorg. Chem. 45, 7261-7268 (2006).
152. D. A. Atwood, J. Delcamp, M. K. Zaman, “Synthesis of 1,3-bis(4,5-dihydrothiazole) benzene” Main Group Chem., 5, 137-140 (2006).
153. A. Mitra, L. J. DePue, J. E. Struss, B. P. Patel, S. Parkin, D. A. Atwood, “Mononuclear Schiff Base Boron Halides: Synthesis, Characterization, and Dealkylation of Trimethyl Phosphate” Inorg. Chem. 45, 9213-9224 (2006).
154. K. M. Zaman, L. Y. Blue, F. E. Huggins, D. A. Atwood, “Cd, Hg, and Pb Compounds of Benzene-1,3-diamidoethanethiol (BDETH2)”, Inorg. Chem. 46, 1975-1980 (2007).
155. K. M. Zaman, C. Chusuei, L. Y. Blue, D. A. Atwood “Prevention of Sulfide Mineral Leaching Through Covalent Coating” Main Group Chem. 6, 169-184 (2007).
156. A. Mitra, S. Parkin, D. Atwood, “Halogen-Containing Tetrametallic Aluminum Alkoxides” J. Chem. Soc.Dalton Trans. 1037-1042 (2008).
157. A. Mitra, D. A. Atwood, J. Struss, D. J. Williams, B. J. McKinney, W. R. Creasy, D. J. McGarvey, H. D. Durst, R. Fry, “Group 13 Chelates in Nerve Gas Agent and Pesticide Dealkylation” New J. Chem., 32, 783-785 (2008).
158. L. Y. Blue, M. A. Van Aelstyn, M. A. Matlock, M, D. A. Atwood, “Low-Level Mercury Removal from Groundwater Using a Synthetic Chelating Ligand” Wat. Res.,42, 2025-2028 (2008).
159. S. Dagorne, and D. A. Atwood, “The Synthesis, Characterization, and Applications of Group 13 Cationic Compounds” Chem. Rev. 108, 4037-4071 (2008).
160. C. C. Chusuei, K. M. Zaman, D. A. Atwood, “Charge Transfer Between Benzene-1,3-diamidoethanethiol (BDET) and Metal Sulfides Affect Efficiency of Acid Mine Drainage Treatment” Colloids and Surfaces A: Physicochem. Eng. Aspects 331, 155-161 (2008).
161. A. Hutchison, D. Atwood, Q. Eduardo Santillian-Jimenez, “The Removal of Mercury from Water by Open Chain Ligands Containing Multiple Sulfurs” J. Haz. Mat., 156, 458-465 (2008).
162. L. Y. Blue, P. Jana, D. A. Atwood, “Aqueous Mercury Precipitation with the Synthetic Dithiolate, BDTH2” Fuel 89, 1326-1330 (2009)
163. L. Hewitson, L. A. Houser, C. Stott, G. Sackett, J. L. Tomko, D. Atwood, L. Blue, E. R. White, A. J. Wakefield, “Delayed Acquisition of Neonatal Reflexes in Newborn Primates Receiving a Thimerosal-Containing Hepatitis B Vaccine: Influence of Gestational Age and Birth Weight” J. Toxicology and Env. Health, Part A, 73, 1298-1313 (2010).
164. A. Mitra, B. C. Yearwood, T. S. Keizer, D. A. Atwood, “Oxide Compound Formation between Boron and Excess Magnesium” Main Group Chem. 9, 193-201 (2010).
165. R. Butala, J. K. Cooper, A. Mitra, M. K. Webster, D. A. Atwood, “Dealkylation of Chemical Weapon Agents and Pesticides with Group 13 Salen Compounds” Main Group Chem. 9, 315-336 (2010).
166. K. Kim, O. G. Tsay, D. A. Atwood, D. G. Churchill, “Destruction and Detection of Chemical Warfare Agents” Chem. Rev. 111, 5345-5403 (2011).
167. Removal of Mercury from the Environment – A Quantum Chemical Study with the Normalized Elimination of the Small Component (NESC) Method, Zou, W.; Filatov, M.; Atwood, D.; Cremer, D., Inorg. Chem. 52 (2013) 2497-2505.
168. C6H4S2AsCl: Description and Interpretation of an Incommensurately Modulated Molecular Crystal Structure, Bakus II, R.C.; Atwood, D.; Parkin, S.; Brock, C. P.; Petříček, V. Acta Cryst. B69 (2013) 496-508.
169. Removal of Selenite from Water Using a Synthetic Dithiolate: An Experimental and Quantum Chemical Investigation, Burriss, D.; Zou, W.; Cremer, D.; Walrod, J.; Atwood, D.; Inorg. Chem. 53 (2014) 4010-4021.
170. Lewis Acid-Assisted Detection of Nerve Agents in Water, Butala, R. R.; Creasy, W. R.; Fry, R. A.; McKee, M. L.; Atwood, D. A. Chem. Commun. 51 (2015) 9269-9271.
171. Quality of Water from Tile Drains in Fields Treated with Poultry Litter in McLean County, Kentucky, Beck, E. Glynn; Blue, Lisa Y.; Atwood, D. A. Kentucky Geological Survey, Information Circular 32, Series XII, fall 2015.
- "Synthesis, Characterization, and Stability of Dealkylated Salen-Supported Aluminum Phosphates."Inorganic chemistry60.7(2021):4456-4462.Details. Full text
- "Self-Assembly of a Semiconductive and Photoactive Heterobimetallic Metal-Organic Capsule."Angewandte Chemie (International ed. in English)60.19(2021):10516-10520.Details. Full text
- "Controlled hierarchical self-assembly of networked coordination nanocapsules <i>via</i> the use of molecular chaperones."Chemical science11.46(2020):12547-12552.Details. Full text
- "Biomimetic Self-Assembly of Co<sup>II</sup>-Seamed Hexameric Metal-Organic Nanocapsules."Journal of the American Chemical Society141.23(2019):9151-9154.Details. Full text
- "Update 1 of: Destruction and Detection of Chemical Warfare Agents."Chemical reviews115.24(2015):PR1-76.Details. Full text
- "Lewis acid-assisted detection of nerve agents in water."Chemical communications (Cambridge, England)51.45(2015):9269-71.Details. Full text
- "C6H4S2AsCl: description and interpretation of an incommensurately modulated molecular crystal structure."Acta crystallographica Section B, Structural science, crystal engineering and materials69.Pt 5(2013):496-508.Details. Full text
- "Low-level mercury removal from groundwater using a synthetic chelating ligand."Water research42.8-9(2008):2025-8.Details. Full text
- "Cd, Hg, and Pb Compounds of Benzene-1,3-diamidoethanethiol (BDETH(2))."Inorganic chemistry46.6(2007):1975-80.Details. Full text
- "Mononuclear Schiff base boron halides: synthesis, characterization, and dealkylation of trimethyl phosphate."Inorganic chemistry45.23(2006):9213-24.Details. Full text
- "Five-coordinate aluminum bromides: synthesis, structure, cation formation, and cleavage of phosphate ester bonds."Journal of the American Chemical Society128.4(2006):1147-53.Details. Full text
- "Mercury(II) 2-aminoethanethiolate clusters: intramolecular transformations and mechanisms."Inorganic chemistry45.18(2006):7261-8.Details. Full text
- "Aluminum phosphinate and phosphates of salen ligands."Inorganic chemistry45.10(2006):3970-5.Details. Full text
- "Solution and solid-state study of heteroleptic Hg(II)-thiolates: crystal structures of Hg4I4(SCH2CH2NH2)4 and Hg4I8(SCH2CH2NH3)2 n.nH2O."Inorganic chemistry45.5(2006):2112-8.Details. Full text
- "Mercurophilic interaction in novel polynuclear Hg(II)-2-aminoethanethiolates."Dalton transactions (Cambridge, England : 2003)24(2005):3874-80.Details. Full text
- "Structure-directing influence of halide in mercury thiolate clusters."Inorganic chemistry44.16(2005):5753-60.Details. Full text
- "Soft metal preferences of 1,3-benzenediamidoethanethiol."Water research37.3(2003):579-84.Details.
- "Salen-type compounds of calcium and strontium."Inorganic chemistry41.21(2002):5397-402.Details. Full text
- "Advanced mercury removal from gold leachate solutions prior to gold and silver extraction: a field study from an active gold mine in Peru."Environmental science & technology36.7(2002):1636-9.Details. Full text
- "Effectiveness of commercial reagents for heavy metal removal from water with new insights for future chelate designs."Journal of hazardous materials92.2(2002):129-42.Details. Full text
- "Catalytic dealkylation of phosphates with binuclear boron compounds."Journal of the American Chemical Society124.9(2002):1864-5.Details. Full text
- "Chemical precipitation of heavy metals from acid mine drainage."Water research36.19(2002):4757-64.Details. Full text
- "Monomeric uni-ligated aluminates."Chemical communications (Cambridge, England)20(2001):2094-5.Details. Full text
- "Reactivity and derivatization of five-coordinate, chelated aluminum."Inorganic chemistry40.26(2001):6782-7.Details. Full text
- "Group 13 compounds incorporating Salen ligands."Chemical reviews101.1(2001):37-52.Details. Full text
- "Aqueous leaching properties and environmental implications of cadmium, lead and zinc trimercaptotriazine (TMT) compounds."Water research35.15(2001):3649-55.Details. Full text
- "Chemistry of 2,4,6-trimercapto-1,3,5-triazine (TMT): acid dissociation constants and group 2 complexes."Inorganic chemistry40.17(2001):4443-7.Details. Full text
- "Irreversible precipitation of mercury and lead."Journal of hazardous materials84.1(2001):73-82.Details. Full text
- "A pyridine-thiol ligand with multiple bonding sites for heavy metal precipitation."Journal of hazardous materials82.1(2001):55-63.Details. Full text